Kwimveliso kunye nobomi, i-silica gel ingasetyenziselwa ukomisa i-N2, umoya, i-hydrogen, igesi yendalo [1] njalo njalo.Ngokutsho kwe-asidi kunye ne-alkali, i-desiccant inokwahlulwa ibe yi-acid desiccant, i-alkaline desiccant kunye ne-desiccant engathathi hlangothi [2].Ijeli ye-silica ibonakala iyinto eyomileyo engathathi hlangothi ebonakala yomile i-NH3, i-HCl, i-SO2, njl. kwaye umphezulu utyebile kumaqela e-hydroxyl (jonga uMfanekiso 1).Isizathu sokuba ijeli ye-silica ikwazi ukufunxa amanzi kukuba iqela le-silicon hydroxyl kumphezulu we-silica gel linokwenza i-intermolecular hydrogen bonds kunye neemolekyuli zamanzi, ngoko ke inokubhengeza amanzi kwaye ngaloo ndlela idlale indima yokomisa.Ijeli ye-silica eguqula umbala iqulethe i-cobalt ion, kwaye emva kokuba amanzi e-adsorption afikelele kwi-saturation, i-cobalt ion kwi-silica gel eguqula umbala ibe yi-hydrated cobalt ion, ukuze i-gel silica eluhlaza ibe yipinki.Emva kokufudumeza ijeli ye-silica epinki kwi-200 ℃ kangangexesha elithile, ibhondi ye-hydrogen phakathi kwejeli ye-silica kunye neemolekyuli zamanzi iyaphuka, kwaye ijeli ye-silica eguquliweyo iya kujika ibe luhlaza kwakhona, ukuze umzobo wesakhiwo se-silicic acid kunye nejeli ye-silica ikwazi. isetyenziswe kwakhona njengoko kuboniswe kuMfanekiso 1. Ngoko, ekubeni umphezulu we-silica gel ucebile kumaqela e-hydroxyl, umphezulu we-silica gel unokwenza i-intermolecular hydrogen bond kunye ne-NH3 kunye ne-HCl, njl., kwaye kungabikho ndlela yokwenza njenge i-desiccant ye-NH3 kunye ne-HCl, kwaye akukho ngxelo efanelekileyo kwiincwadi ezikhoyo.Yaba yintoni ke imiphumo?Lo mbandela wenze olu phando lulandelayo lovavanyo.
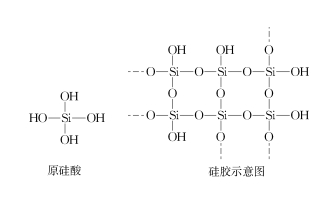
IKHIWANE.1 Umzobo wesakhiwo se-ortho-silicic acid kunye ne-silica gel
2 iCandelo loVavanyo
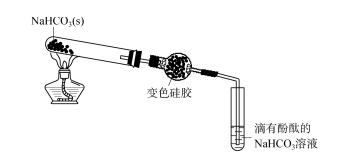
2.1 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - i-Ammonia Okokuqala, i-gel ye-silica e-discolored yafakwa emanzini adibeneyo kunye ne-ammonia egxininiswe ngokulandelanayo.Ijeli ye-silica eguqulwe umbala ijika ibepinki kumanzi adityanisiweyo;Kwi-ammonia egxininisiweyo, i-silicone etshintsha umbala kuqala ijike ibe bomvu kwaye kancinci ijike luhlaza okwesibhakabhaka.Oku kubonisa ukuba ijeli ye-silica inokufunxa i-NH3 okanye i-NH3 ·H2 O kwi-ammonia.Njengoko kuboniswe kwi-Figure 2, i-calcium hydroxide eqinile kunye ne-ammonium chloride ixutywe ngokulinganayo kwaye ishushu kwi-tube yokuvavanya.Irhasi evelayo isuswa ngekalika yealkali emva koko ngejeli yesilica.Umbala wejeli ye-silica kufuphi nendlela yokungena uya uba khaphukhaphu (umbala wesicelo somda we-silica gel desiccant kuMzobo 2 uphononongwa - i-ammonia 73, isigaba se-8 sika-2023 ngokusisiseko siyafana nombala wejeli ye-silica efakwe emanzini. kumanzi ammonia agxininisiweyo), kwaye iphepha lovavanyo lwe-pH alikho utshintsho olucacileyo.Oku kubonisa ukuba i-NH3 eveliswayo ayifikanga kwiphepha lovavanyo lwe-pH, kwaye sele ibhengezwe ngokupheleleyo.Emva kwexesha elithile, yeka ukufudumeza, ukhuphe inxalenye encinci yebhola ye-silica gel, uyibeke emanzini adityanisiweyo, yongeza i-phenolphthalein emanzini, isisombululo sijika sibe bomvu, esibonisa ukuba ijeli ye-silica inefuthe elinamandla le-adsorption. I-NH3, emva kokuba amanzi adibeneyo avaliweyo, i-NH3 ingena emanzini adibeneyo, isisombululo si-alkaline.Ngoko ke, ngenxa yokuba i-silica gel ine-adsorption eyomeleleyo ye-NH3, i-agent yokomisa i-silicone ayikwazi ukomisa i-NH3.
IKHIWANE.2 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - ammonia
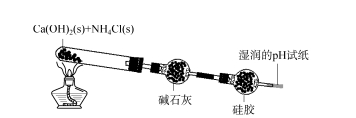
2.2 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - i-hydrogen chloride kuqala itshisa i-NaCl solids kunye ne-alcohol flame flame ukususa amanzi amanzi kwiindawo eziqinileyo.Emva kokuba isampuli ipholile, i-asidi ye-sulfuric egxininisiweyo yongezwa kwi-NaCl solids ukuvelisa ngokukhawuleza inani elikhulu lamaqamza.I-gas eveliswayo idluliselwa kwi-tube yokumisa i-spherical ene-silica gel, kwaye iphepha lokuvavanya i-pH emanzi libekwe ekupheleni kombhobho wokumisa.Ijeli ye-silica ngaphambili ijika ibe luhlaza okhanyayo, kwaye iphepha lokuvavanya i-pH emanzi alikho utshintsho olucacileyo (jonga uMfanekiso 3).Oku kubonisa ukuba igesi yeHCl eyenziwe ibhengezwa ngokupheleleyo yijeli ye-silica kwaye ayiphumi emoyeni.
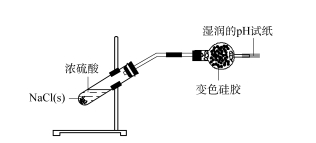
Umfanekiso wesi-3 Uphando kububanzi bokusetyenziswa kwe-silica gel desiccant - i-hydrogen chloride
Ijeli ye-silica idsorbed i-HCl kwaye yajika yaluhlaza yafakwa kwityhubhu yovavanyo.Faka ijeli entsha eluhlaza okwesibhakabhaka kwityhubhu yovavanyo, yongeza i-hydrochloric acid egxininisiweyo, ijeli ye-silica nayo iba nombala oluhlaza okhanyayo, imibala emibini iyafana.Oku kubonisa igesi yejeli yesilica kwityhubhu yokomisa engqukuva.
2.3 Ukuphononongwa komlinganiselo wesicelo se-silica gel desiccant - i-sulfur dioxide I-Mixed concentrated sulfuric acid kunye ne-sodium thiosulfate eqinile (jonga umfanekiso 4), i-NA2s2 O3 + H2 SO4 ==Na2 SO4 + SO2 ↑+S↓+H2 O;Irhasi eveliswayo igqithiswa kwityhubhu yokumisa equlethe ijeli ye-silica eguqulweyo, ijeli ye-silica eguqulweyo iba buhlaza okwesibhakabhaka, kwaye iphepha eliluhlaza okwesibhakabhaka ekupheleni kwephepha lovavanyo elimanzi alitshintshi kakhulu, libonisa ukuba igesi ye-SO2 eyenziweyo Ithengiswe ngokupheleleyo yibhola yejeli yesilica kwaye ayinakubaleka.
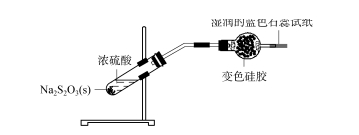
IKHIWANE.4 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - i-sulfur dioxide
Susa inxalenye yebhola yejeli ye-silica kwaye uyibeke emanzini adibeneyo.Emva kokulinganisela okupheleleyo, thabatha ithontsi elincinci lamanzi kwiphepha eliluhlaza okwesibhakabhaka le-litmus.Iphepha lokuvavanya alitshintshi kakhulu, libonisa ukuba amanzi adiyiweyo awanele ukuba adibanise i-SO2 kwi-silica gel.Thatha inxalenye encinci yebhola ye-silica gel kwaye uyitshise kwi-tube yokuvavanya.Beka iphepha elimanzi le-litmus emlonyeni wetyhubhu yovavanyo.Iphepha le-litmus eluhlaza lijika libe bomvu, libonisa ukuba ukufudumeza kwenza igesi ye-SO2 ikhutshwe kwibhola ye-silica gel, ngaloo ndlela yenza iphepha le-litmus libe bomvu.Olu vavanyo lungentla lubonisa ukuba ijeli ye-silica nayo ineempembelelo ezinamandla ze-adsorption kwi-SO2 okanye i-H2 SO3, kwaye ayinakusetyenziselwa ukomisa igesi ye-SO2.
2.4 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - iCarbon dioxide
Njengoko kubonisiwe kuMfanekiso 5, isisombululo se-sodium bicarbonate esithontsiza i-phenolphthalein sibonakala sibomvu ngokukhanyayo.Isodium bicarbonate eqinileyo iyafudunyezwa kwaye umxube werhasi obangelwayo ugqithiswa kwityhubhu yokomisa equlethe i-silica gel spheres ezomisiweyo.Ijeli ye-silica ayitshintshi kakhulu kwaye i-sodium bicarbonate ethontsiza nephenolphthalein ibhengeza iHCl.I-cobalt ion kwijeli ye-silica ekhutshiweyo yenza isisombululo esiluhlaza kunye ne-Cl- kwaye ngokuthe ngcembe iba nemibala, ebonisa ukuba kukho i-CO2 eyinkimbinkimbi yegesi ekupheleni kwetyhubhu yokomisa i-spherical.Ijeli ye-silica ekhanyayo-eluhlaza ifakwe emanzini adityanisiweyo, kwaye ijeli ye-silica eguquguqukayo ngokuthe ngcembe itshintsha ibe tyheli, ebonisa ukuba i-HCl ebhengezwa ngejeli ye-silica iye yachithwa emanzini.Isixa esincinci sesisombululo se-aqueous esiphezulu songezwa kwisisombululo se-nitrate yesilivere e-acidified yi-nitric acid ukwenza i-precipitate emhlophe.Isixa esincinci sesisombululo esinamanzi sichithwa kuluhlu olubanzi lwephepha lokuhlola i-pH, kwaye iphepha lokuvavanya lijika libomvu, libonisa ukuba isisombululo si-acidic.Olu vavanyo lungasentla lubonisa ukuba ijeli ye-silica ine-adsorption eyomeleleyo kwigesi yeHCl.I-HCl yimolekyuli ye-polar enamandla, kunye neqela le-hydroxyl kumphezulu we-silica gel nayo ine-polarity eyomeleleyo, kwaye ezi zimbini zinokwenza i-intermolecular hydrogen bond okanye zibe nentsebenziswano eqinile ye-dipole ye-dipole, ekhokelela kumandla anamandla phakathi kwe-intermolecular phakathi komphezulu we-silica. iimolekyuli ze-gel kunye ne-HCl, ngoko i-silica gel ine-adsorption enamandla ye-HCl.Ke ngoko, i-agent yokomisa i-silicone ayinakusetyenziselwa ukomisa ukubaleka kweHCl, oko kukuthi, ijeli ye-silica ayibhengezi i-CO2 okanye i-adsorb kuphela i-CO2.
IKHIWANE.5 Ukuphononongwa kobubanzi bokusetyenziswa kwe-silica gel desiccant - carbon dioxide
Ukuze kuqinisekiswe i-adsorption ye-silica gel kwi-carbon dioxide gas, le mifuniselo elandelayo iyaqhubeka.Ibhola yejeli ye-silica kwityhubhu yokomisa engqukuva yasuswa, kwaye inxalenye yahlulahlulwe yaba sisisombululo sesodium bicarbonate ethontsiza phenolphthalein.Isisombululo se-sodium bicarbonate sathotywa umbala.Oku kubonisa ukuba ijeli ye-silica ibhengeza i-carbon dioxide, kwaye emva kokunyibilika emanzini, i-carbon dioxide desorbs kwisisombululo se-sodium bicarbonate, yenza isisombululo se-sodium bicarbonate.Inxalenye eseleyo yebhola ye-silicone ishushu kwityhubhu yokuvavanya eyomileyo, kwaye igesi ebangelwayo idluliselwa kwisisombululo se-sodium bicarbonate evuzayo ngephenolphthalein.Kungekudala, isisombululo se-sodium bicarbonate sitshintsha ukusuka ekukhanyeni okubomvu ukuya kumbala ongenambala.Oku kukwabonisa ukuba ijeli ye-silica isenawo amandla e-adsorption yegesi ye-CO2.Nangona kunjalo, i-adsorption force ye-silica gel kwi-CO2 incinci kakhulu kune-HCl, i-NH3 kunye ne-SO2, kunye ne-carbon dioxide ingabhengezwa ngokuyinxenye ngexesha lovavanyo ku-Figure 5. ukuba ijeli ye-silica kunye ne-CO2 zenza i-intermolecular hydrogen bonds Si - OH ... O =C.Ngenxa yokuba i-athomu ye-carbon ephakathi ye-CO2 yi-sp hybrid, kunye ne-athomu ye-silicon kwijeli ye-silica yi-sp3 hybrid, i-molecule ye-CO2 ehambelanayo ayisebenzisani kakuhle nomphezulu wejeli ye-silica, okukhokelela kumandla we-adsorption ye-silica gel kwi-carbon dioxide. encinci.
3.Ukuthelekisa phakathi kwe-solubility yeegesi ezine emanzini kunye nesimo se-adsorption phezu kobuso be-silica gel Ukususela kwiziphumo zovavanyo ezingentla, kunokubonwa ukuba i-silica gel inomthamo onamandla we-adsorption we-ammonia, i-hydrogen chloride kunye ne-sulfur dioxide, kodwa amandla amancinci e-adsorption ye-carbon dioxide (jonga iTheyibhile 1).Oku kufana nokunyibilika kweegesi ezine emanzini.Oku kungenxa yokuba iimolekyuli zamanzi ziqulethe i-hydroxy-OH, kwaye umphezulu we-silica gel ucebile kwi-hydroxyl, ngoko ke ukunyibilika kwezi gesi ezine emanzini kufana kakhulu nokufakwa kwayo kumphezulu we-silica gel.Phakathi kweegesi ezintathu zegesi ye-ammonia, i-hydrogen chloride kunye ne-sulphur dioxide, i-sulphur dioxide ineyona nto incinci yokunyibilika emanzini, kodwa emva kokuba ibhengezwe ngejeli ye-silica, yeyona nto inzima kakhulu ukuyikhupha phakathi kweegesi ezintathu.Emva kokuba i-silica gel ibhengeza i-ammonia kunye ne-hydrogen chloride, inokuchithwa ngamanzi adibeneyo.Emva kokuba igesi yesulfure dioxide ifakwe kwijeli ye-silica, kunzima ukuyikhupha ngamanzi, kwaye kufuneka ifudunyezwe idesorption ukusuka kumphezulu wejeli ye-silica.Ngoko ke, i-adsorption yeegesi ezine kumphezulu we-silica gel kufuneka ibalwe ngokwethiyori.
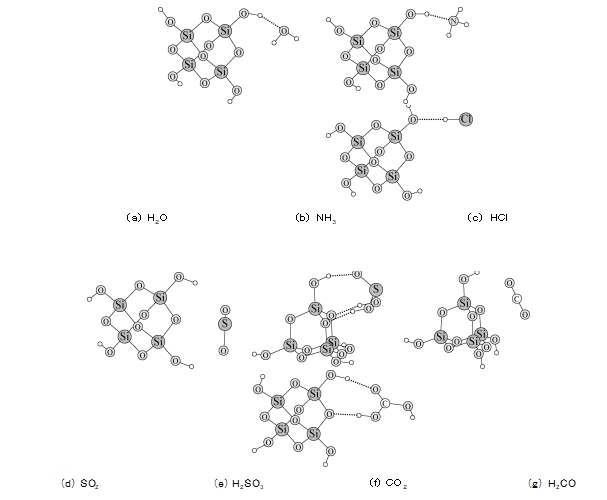
I-4 Ukubala kwethiyori yokusebenzisana phakathi kwe-silica gel kunye neegesi ezine zinikezelwa kwi-quantumization ORCA software [4] phantsi kwesakhelo se-density functional theory (DFT).Indlela ye-DFT D/B3LYP/Def2 TZVP yasetyenziselwa ukubala iindlela zokusebenzisana kunye namandla phakathi kweegesi ezahlukeneyo kunye nejeli ye-silica.Ukuze kube lula ukubala, i-silica gel solids imelwe yi-tetrameric orthosilicic acid molecules.Iziphumo zokubala zibonisa ukuba i-H2 O, NH3 kunye ne-HCl zonke zinokwenza iibhondi ze-hydrogen kunye neqela le-hydroxyl kumphezulu wejeli ye-silica (jonga uMfanekiso 6a ~ c).Banamandla okubopha okuqinileyo kumphezulu we-silica gel (jonga iTheyibhile 2) kwaye zibhengezwa ngokulula kumphezulu wejeli ye-silica.Ekubeni amandla okubopha i-NH3 kunye ne-HCl afana nalawo e-H2 O, ukuhlamba amanzi kunokukhokelela ekukhutshweni kwezi molekyuli ezimbini zegesi.Kwi-molecule ye-SO2, amandla ayo okubopha kuphela -17.47 kJ / mol, encinci kakhulu kunezi molecule ezintathu zingentla.Nangona kunjalo, uvavanyo luqinisekisile ukuba igesi ye-SO2 ibhengezwa ngokulula kwijeli ye-silica, kwaye nokuhlamba akunako ukuyichitha, kwaye ukufudumeza kuphela okunokwenza i-SO2 ibaleke kumphezulu wejeli ye-silica.Ngoko ke, siqikelele ukuba i-SO2 inokudibanisa ne-H2 O kumphezulu we-silica gel ukwenza amaqhezu e-H2 SO3.Umzobo we-6e ubonisa ukuba i-molecule ye-H2 SO3 yenza iibhondi ezintathu ze-hydrogen kunye ne-hydroxyl kunye ne-athomu ze-oksijini kumphezulu we-silica gel ngaxeshanye, kwaye amandla okubopha aphakamileyo njenge-76.63 kJ / mol, echaza ukuba kutheni i-SO2 idsorbed Ijeli ye-silica kunzima ukuyiphepha emanzini.I-CO2 engeyiyo i-polar inamandla okubopha obuthathaka kunye nejeli ye-silica, kwaye inokubhengezwa kuphela ngokuyinxenye yijeli ye-silica.Nangona amandla okubopha i-H2 CO3 kunye ne-silica gel nayo yafikelela -65.65 kJ / mol, izinga lokuguqulwa kwe-CO2 kwi-H2 CO3 lalingekho phezulu, ngoko ke izinga le-adsorption ye-CO2 lancitshiswa.Inokubonwa kwidatha engentla apha ukuba i-polarity ye-molecule yegesi ayiyona kuphela inqobo yokugweba ukuba ingaba i-adsorbed nge-silica gel, kunye ne-hydrogen bond eyenziwe nge-silica gel surface iyona sizathu esibalulekileyo sokubhengezwa kwayo okuzinzile.
Ukubunjwa kwe-silica gel yi-SiO2 · nH2 O, indawo enkulu ye-silica gel kunye neqela elicebileyo le-hydroxyl phezu komhlaba lenza i-silica gel ingasetyenziselwa njengesomisi esingenabuthi esinetyhefu kunye nokusebenza okugqwesileyo, kwaye isetyenziswa ngokubanzi kwimveliso kunye nobomi. .Kweli phepha, kuqinisekiswa kwimiba emibini yovavanyo kunye nokubala kwethiyori ukuba i-silica gel ingabhengeza i-NH3, i-HCl, i-SO2, i-CO2 kunye nezinye iigesi ngokusebenzisa iibhondi ze-hydrogen ze-intermolecular, ngoko i-silica gel ayinakusetyenziselwa ukomisa ezi gesi.Ukubunjwa kwe-silica gel yi-SiO2 · nH2 O, indawo enkulu ye-silica gel kunye neqela elicebileyo le-hydroxyl phezu komhlaba lenza i-silica gel ingasetyenziselwa njengesomisi esingenabuthi esinetyhefu kunye nokusebenza okugqwesileyo, kwaye isetyenziswa ngokubanzi kwimveliso kunye nobomi. .Kweli phepha, kuqinisekiswa kwimiba emibini yovavanyo kunye nokubala kwethiyori ukuba i-silica gel ingabhengeza i-NH3, i-HCl, i-SO2, i-CO2 kunye nezinye iigesi ngokusebenzisa iibhondi ze-hydrogen ze-intermolecular, ngoko i-silica gel ayinakusetyenziselwa ukomisa ezi gesi.
3
IKHIWANE.Iindlela ze-6 zokusebenzisana phakathi kweeamolekyu ezahlukeneyo kunye ne-silica gel surface kubalwa ngendlela ye-DFT
Ixesha lokuposa: Nov-14-2023